New research documents how modifications to RNA keep nerve junctions flexible.
Researchers found that a molecule, called m6A, makes modifications at brain nerve junctions that are essential for proper signal transmission. The research finding, published in the journal Nature Neuroscience , could help further understanding of neurodevelopmental and neuropsychiatric disorders, including autism.
A lot happens at the junctions between nerve fibres in the brain, which are called synapses. For example, chemicals are transmitted across synapses to keep electrical impulses passing from one fibre to the next. Synapses, which include the ends of nerve fibres and the junctions in between, are also constantly changing their structures and transmission efficiency, and being synthesized and degraded. This property of constant change, known as synaptic plasticity, is a fundamental feature of brain function. And all that activity requires protein synthesis by RNA.
Dan Ohtan Wang from Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS) and colleagues wanted to investigate whether N6-methyl-adenosine (m6A) plays a role in modifying the RNA that reads protein code at synapses. The m 6 A molecule is one of the most common and abundant RNA modifiers present in most living organisms, but no one had studied its activities at nerve synapses.
The team, including researchers in Japan and the US, isolated synapses from the forebrains of healthy adult mice and examined the compositions of the RNA molecules present at these synapses.
They found that m6A was attached to around 4,500 sites on almost 3,000 genes on the RNA molecules. These m6A modifications were involved in synapse synthesis and modulation. They were also active in pathways associated with neurodevelopmental and neuropsychiatric disorders.
Preventing m6A modifications led to synaptic dysfunction and dampening of nerve transmission at the synapse, the researchers found. It also led to malformations in protrusions called dendritic spines that were similar to malformations that happen in humans with intellectual impairment.
“Our findings indicate that chemical modifications of synaptic mRNAs [involving m6A] critically contribute to synaptic function,” the researchers conclude.
This work builds on previous studies that found the largest amount of m6A in the body is present in the brain, where it contributes to embryonic brain development, locomotion, circadian clock regulation, and fear memory.
“This study provides the initial draft of the epitranscriptome at synapses and points to a previously unknown molecular regulatory pathway,” says Wang. “The fact that this regulation occurs directly on the RNA localized to synapses may be extremely important in generating the diversity and complexity of synapses that underlies our cognition and behaviour,” she says.
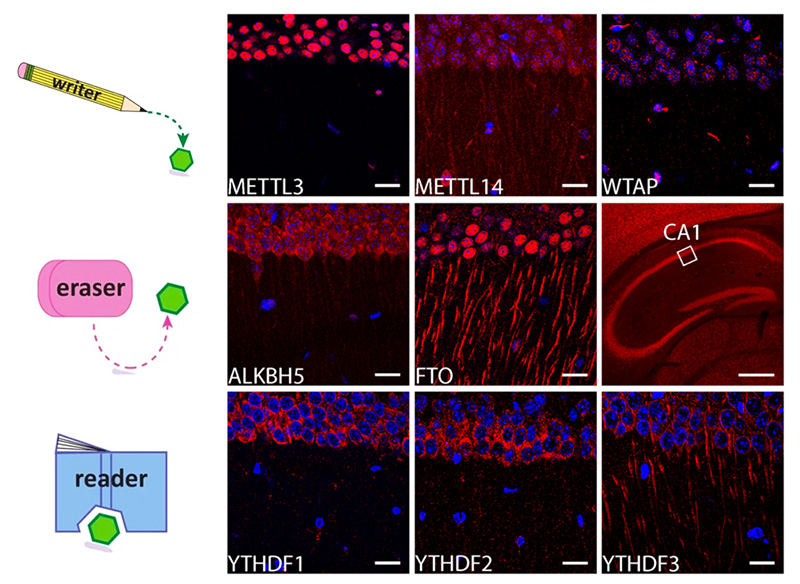
In living mice, the proteins controlled by m6A exist at dendrites of hippocampal neurons. (Top) The proteins that add m6A to mRNA. (Middle) The proteins that remove m6A. (Bottom) The proteins that detect m6A (iCeMS/Ohtan lab)
Paper information
【DOI】 https://doi.org/10.1038/s41593-018-0173-6
Daria Merkurjev, Wan-Ting Hong, Kei Iida, Ikumi Oomoto, Belinda J. Goldie, Hitoshi Yamaguti, Takayuki Ohara, Shin-ya Kawaguchi, Tomoo Hirano, Kelsey C. Martin, Matteo Pellegrini, Dan Ohtan Wang (2018). Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nature Neuroscience, 21(7), 1004-1014.





