
Fibrodysplasia ossificans progressive (FOP) is a rare, but devastating genetic disease where bone is grown within soft tissue, such as skeletal muscle. Accordingly, it is also known as Stone Man Syndrome, since the patient's body ossifies into a statue-like state. It is a genetic disease, but patients do not show symptoms until their school-aged years. Normally, an immune response or trauma triggers the disease. This complicates study of FOP because the trauma associated with acquiring patient cells could stimulate the irregular bone growth. For the same reason, surgical treatments that remove excessive bone are not an option, because these too will only cause more bone growth in the post-operation healing period.
Induced pluripotent stem (iPS) cells offer an attractive model to study the disease, because researchers can take cells from the patient that are unaffected by FOP and then reprogram the cells into soft tissue cells for study. Researchers at the Center for iPS Cell Research and Application (CiRA), Kyoto University, took advantage of this strategy by reprogramming FOP patient cells and then seeking candidate molecules that could explain how the disease initiates. The researchers focused on bone morphogenetic proteins (BMP). BMP are what stimulate bone healing after a fracture or break. However, in FOP patients, BMP signaling appears hyperactive. "There are two popular theories," explains Makoto Ikeya, an associate professor at CiRA involved in the study. "In one, BMP signaling is always active. In the other, BMP signaling is abnormally strong when activated."
For the purpose of FOP drug discovery, scientists have normally focused on molecules associated with BMP signaling. However, CiRA researchers from Professor Junya Tochuchda's lab instead looked at molecules related with inflammation, finding Activin-A as a candidate drug target. "Because patients normally show FOP symptoms after trauma or inflammation, we thought this would be a good strategy," Ikeya said. Using iPS cell technology, the scientists found that only cells harboring the FOP gene mutation would respond to Activin-A by significantly increasing their BMP signaling. Further, transplanting these cells into mice and stimulating them with Activin-A led to abnormal bone.
According to another author of the study, CiRA Professor Junya Toguchida, the discovered relationship between inflammation and bone formation gives a whole new perspective on treatments. "Our model provides a new platform for drug discovery and the study of bone formation that did not exist before," he said.
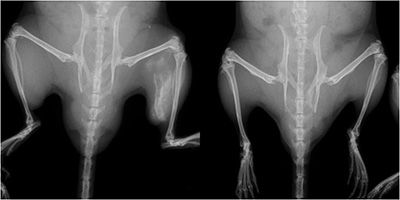
FOP mouse (left) and normal mouse. The FOP mouse shows abnormal bone growth in the left hind leg. CiRA scientists use iPS cells to show that a molecule associated with inflammation could be a therapeutic target for the abnormal bone growth.
Paper Information
[DOI] http://dx.doi.org/10.1073/pnas.1510540112
Kyosuke Hino, Makoto Ikeya, Kazuhiko Horigome, Yoshihisa Matsumoto, Hayao Ebise, Megumi Nishio, Kazuya Sekiguchi, Mitsuaki Shibata, Sanae Nagata, Shuichi Matsuda, and Junya Toguchida
"Neofunction of ACVR1 in fibrodysplasia ossificans progressiva"
PNAS , published ahead of print on 30 November 2015





