Heart failure, which is often the final outcome of various cardiovascular diseases has a poor prognosis. It is known that abnormalities in cardiomyocyte intracellular calcium ion regulation are deeply involved in this pathology that impairs the contraction and relaxation of the myocardium.
Heart disease is the leading cause of death worldwide and heart failure accounts for the highest proportion of heart diseases. With an aging society, the number of patients with heart failure is expected to increase. Despite recent advances in treatment, heart failure is still a disease with a poor prognosis. Therefore, there is an urgent need to identify new therapeutic targets and develop new therapeutic methods by clarifying the mechanism underlying the onset and progression of heart failure.
Calcium ion regulation in myocardial cells involves L-type calcium channels (LTCCs), which allow calcium ions to pass from the outside to inside the cells, and calcium ion mobilization via the ryanodine receptor 2 (RyR2) of the myocardial sarcoplasmic reticulum (SR), which stores calcium ions in the cell. It is known that the connection between LTCC and SR in the membrane structure called T-tubule present in the myocardium is important for normal myocardial contraction.
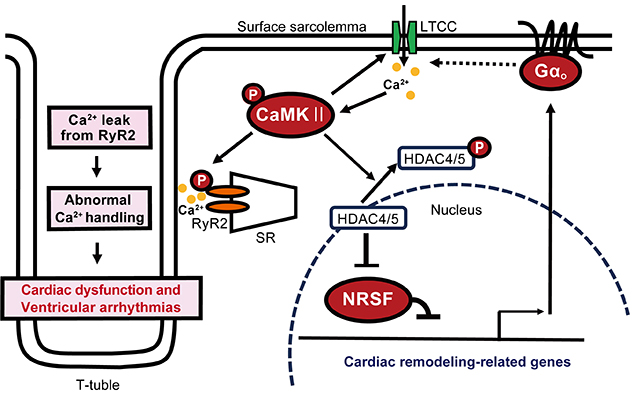
In heart failure, these calcium ion recruitment mechanisms are abnormal, such as decreased LTCC activity in T-tubules, increased LTCC activity in other surface cell membranes, and calcium calmodulin-dependent protein kinase II (CaMKII). It is known that pathological calcium signaling is activated to cause phosphorylation of RyR2 on SR and impair its normal function. The detailed molecular mechanisms involved in the formation of these pathologies had been previously unresolved.
In this study, Assistant Professor Yasuaki Nakagawa, Department of Cardiovascular Science, Graduate School of Medicine, Kyoto University, Professor Koichiro Kuwahara, Department of Cardiovascular Science, Shinshu University, and Professor Mitsuhiko Yamada, Department of Molecular Pharmacology, Shinshu University, with researchers from Michigan University and Juntendo University found that suppression of the expression of GNAO1, a gene regulated by the transcriptional repressor NRSF and upregulation in dysfunctional hearts, improves its pathology in multiple mouse heart failure models, and conversely, overexpression of GNAO1 in the myocardium causes cardiac dysfunction.
In addition, as the mechanism by which GNAO1 is involved in heart failure, enhanced expression of the protein Gαo encoded by GNAO1 increases the activity of LTCC in the myocardial surface cell membrane, thereby activating the pathological calcium signaling pathway in cardiomyocytes and leading to cardiac dysfunction. The group clarified that the upregulation of Gαo in the myocardium due to pathological stress is involved in the progression of heart failure. This is expected to be useful for the development of new therapeutic agents for heart failure. The results of this study were published online in the academic journal "Circulation Research" on December 8, 2021.
Focusing on genes whose expression is upregulated in the heart of heart failure, Prof. Kuwahara had previously discovered the significance of the transcriptional repressor neuron-restrictive silencer factor (NRSF) as a factor that controls the expression of these genes (Kuwahara K, et al. EMBO J 2003). Through analysis of myocardial-specific NRSF knockout mice and myocardial-specific dominant-suppressing mutant NRSF overexpressing mice, both of which present with heart failure, the group found that GNAO1, a gene regulated by NRSF, is upregulated in the ventricle of these mice. It was confirmed that suppression of GNAO1 expression by knockout mice ameliorates the pathological condition in multiple heart failure model mice including the above mice, and conversely, overexpression of GNAO1 in the myocardium is sufficient to cause cardiac dysfunction.
The researchers clarified that the increased expression of Gαo in the myocardium due to pathological stress plays an important role in the progression of cardiac dysfunction and heart failure. The mechanism is that increased expression of Gαo increases the localized activity of L-type calcium channels in the myocardial surface sarcolemma, thereby activating the pathological calcium signaling pathway in myocardial cells and subsequently disrupting the constancy of calcium ion regulation.
This research elucidated a novel mechanism behind the progression of heart failure and furthers understanding of the pathophysiology of heart failure, which is rapidly increasing due to the aging society. This study may also lead to the development of new therapeutic agents for heart failure targeting Gαo, which is still a disease syndrome with a poor prognosis.
DOI: https://doi.org/10.1161/CIRCRESAHA.121.318898
Hideaki Inazumi, Koichiro Kuwahara, Yasuaki Nakagawa, Yoshihiro Kuwabara, Takuro Numaga-Tomita, Toshihide Kashihara, Tsutomu Nakada, Nagomi Kurebayashi, Miku Oya, Miki Nonaka, Masami Sugihara, Hideyuki Kinoshita, Kenji Moriuchi, Hiromu Yanagisawa, Toshio Nishikimi, Hirohiko Motoki, Mitsuhiko Yamada, Sachio Morimoto, Kinya Otsu, Richard M Mortensen, Kazuwa Nakao, Takeshi Kimura (2022). NRSF-GNAO1 Pathway Contributes to the Regulation of Cardiac Ca2+ Homeostasis. Circulation Research, 130(2), 234-248.





