Deconstructing the genetic protocol for isoquinoline alkaloid synthesis
Isoquinoline alkaloids are the basis of numerous analgesics such as morphine and codeine, along with several anti-microbial agents. In a study in Plant and Cell Physiology , the laboratory of Fumihiko Sato, in collaboration with Japan's National Institute for Genetics and the Kazusa DNA Research Institute, characterizes the function of cytochrome P450 genes in the synthesis of isoquinoline alkaloids in the California poppy.
The research gives molecular-level insight into how plants produce a diverse range of metabolites that can be applied to drug synthesis.
In response to infection, predation, and other harm, land plants have evolved the means to generate specialized metabolites. While primarily synthesized for protection, researchers have found that many of these metabolites can be utilized in new drugs, drawing scientific interest in the biosynthetic pathways that lead to the metabolite production.
" California poppies contains more enriched P450 genes involved in isoquinoline alkaloid synthesis compared with other plants, " says Sato. "Macarpine biosynthesis is especially interesting because its biosynthetic pathway involves six P450s."
P450s are a large family of over 50,000 enzymes found in almost all living species. The researchers prepared a draft genome that represented greater than 95% the total genome of the California poppy, and found three families of P450 genes: CYP80, CYP82, and CYP719.
Although the whole genome would be preferable, " Our findings indicate the draft genome sequence is sufficient for genome-mining analysis. The CYP82 genes were rich in uncharacterized biosynthetic enzyme genes involved in isoquinoline alkaloid synthesis," notes Sato.
Further analysis separated the California poppy cells into two groups that produced macarpine at significantly different levels. The determinant was the relative expression of two genes, CYP82P2 and CYP82P3, that affected the expression of the enzyme dihydrosanguinarine 10-hydroxylase.
Naturally, in portions of the plant where this relative expression was low, such as in the roots, so too was the production of macarpine.
Sato emphasizes that detailed understanding of how plants make isoquinoline alkaloids like macarpine will have significant benefit for society.
"Learning about the genes responsible for isoquinoline alkaloid biosynthesis will help us make new drugs," he says.
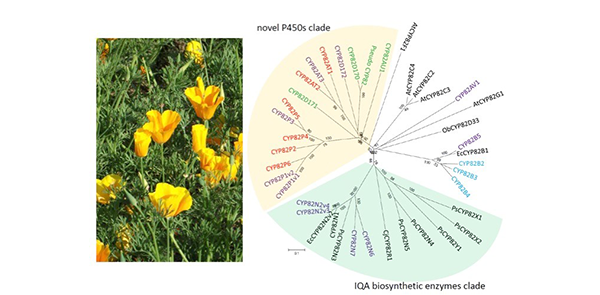
California poppy has diversified cytochrome P450 CYP82 family (Kyoto University / Sato Lab)
Paper information
【DOI】 https://doi.org/10.1093/pcp/pcx210
【KURENAI ACCESS URL】 http://hdl.handle.net/2433/229010
Kentaro Hori, Yasuyuki Yamada, Ratmoyo Purwanto, Yohei Minakuchi, Atsushi Toyoda, Hideki Hirakawa, Fumihiko Sato (2018) Mining of the Uncharacterized Cytochrome P450 Genes Involved in Alkaloid Biosynthesis in California Poppy Using a Draft Genome Sequence. Plant and Cell Physiology, pcx210.





