Kyoto University scientists use fullerenes to contain single molecules of acid
Researchers at Kyoto University's Institute for Chemical Research have developed a method to trap and analyze a hydrated acid using C 70 fullerene cages.
One of the most important chemical processes in nature is the dissociation of an acid in aqueous media. However, the detailed mechanism at the molecular level of acid dissociation and the nature of protons in an aqueous environment (after solvation of the charged fragment) are complex and poorly understood.
The smallest known acid -- hydrogen fluoride (HF) -- has been well studied. However, due to the strong hydrogen bond affinity of HF to H 2 O, it forms many types of oligomers in equilibrium. As a result, the separation and isolation of aqueous HF has been impossible.
To elucidate the intrinsic nature of the hydrated HF molecule, lead author Yasujiro Murata and his team designed a method to contain it in a subnano-sized space, with an inert atmosphere to prevent it from interacting and/or reacting with the surrounding environment. Such spaces can be found within the hollow spheres of fullerenes.
The researchers subjected an open-caged fullerene C 70 derivative to high pressure, in the presence of HF and H 2 O, succeeding in encapsulating H 2 O, HF, and most importantly, H 2 O-HF. The structure of the caged H 2 O-HF complex was then analyzed with single crystal X-ray diffraction, and later nuclear magnetic resonance (NMR) measurements revealed the formation of a hydrogen bond between the H 2 O and HF molecules without proton transfer, even at 140℃.
Murata and his colleagues hope these findings will advance the study of hydrated acids, as well as further the utility of fullerenes in other research. Their results were published recently in the journal Science Advances .
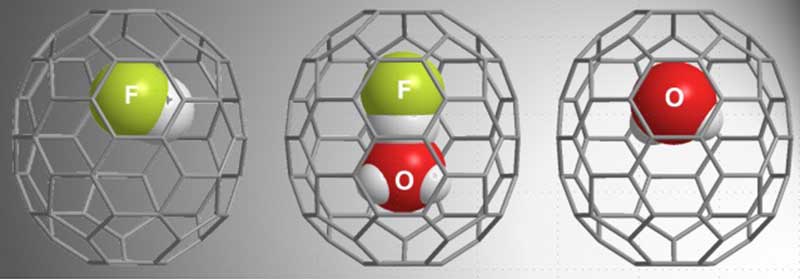
From left, HF molecule, H 2 O-HF complex, and H 2 O molecule contained in C 70 fullerene cages
Paper Information
【DOI】 https://doi.org/10.1126/sciadv.1602833
【KURENAI ACCESS URL】 http://hdl.handle.net/2433/220440
Rui Zhang, Michihisa Murata, Atsushi Wakamiya, Takafumi Shimoaka, Takeshi Hasegawa and Yasujiro Murata (2017). Isolation of the simplest hydrated acid. Science Advances, 3(4), e1602833.





