July 18, 2013
Associate Professor Takayuki Miyazawa, Postdoctoral Fellow Yuki Nakaya from Laboratory of Signal Transduction, Institute for Virus Research, Professor Kazuyoshi Hashizume from Laboratory of Veterinary Physiology, Iwate University, Postdoctoral Fellow Katsuo Koshi from Laboratory of Veterinary Physiology, Iwate University and Postdoctoral Fellow So Nakagawa, Center for Information Biology, National Institute of Genetics, revealed that a bovine endogenous retroviral envelope, termed Fematrin-1, is involved in the Bovinae placentation and has contributed to generating placentation diversity through ruminant evolution.
This study was published in an online version of the Journal of Virology on July 17, 2013.
Outline
During placentation, mammals employ different strategies for nourishing and supporting fetuses. The Bovidae family, consisting of cloven-hoofed ruminants, utilize multiple maternal attachment points on the placenta known as cotyledons, and hybrid cells, named trinucleate cells (TNCs) or syncytial plaques (SyPs), made up of a fusion of fetal trophoblasts and maternal endometrial cells to provide essential hormones and maintain long gestation periods. These hybrid cells are unique to Bovidae, as fetomaternal borders are clearly separated by syncytiotrophoblasts or epithelial cells in the placenta of other mammals. There are three types of trophoblasts known in the bovine placenta: mononucleate trophoblast cells (MTCs), binucleate cells (BNCs) and TNCs. Whereas BNCs are formed by differentiation of MTCs by endoreduplication, TNCs and SyPs are thought to be the result of cell-to-cell fusions between BNCs and maternal endometrial cells. Recently, it was reported that Syncytin-Rum1 was inserted into ruminant genomes including cattle and sheep and possibly involved in fetomaternal cell-to-cell fusion in both species. However, Syncytin-Rum1 alone is insufficient to explain the morphological diversity of the fetomaternal hybrids between Bovinae and Caprinae (i.e., trinucleate cells in Bovinae and syncytial plaques in Caprinae). Here we report that the bovine endogenous retrovirus K1 (BERV-K1) envelope, which we term Fematrin-1, was specifically expressed in BNCs (Figure 1 right panel) throughout gestation in cattle, and induced fusion with bovine endometrial cells in vitro (Fig. 1 left panel) at a significantly higher level than Syncytin-Rum1 under physiological conditions. Fematrin-1 was found to be integrated into intron 18 of FAT tumor suppressor homolog 2 (FAT2) about 18.3 to 25.4 million years ago and has been subject to purifying selection through the evolution of Bovinae (Fig. 2). Phylogenetically, Fematrin-1 is distinct from Syncytins found in other mammalian species that form syncytiotrophoblasts. Our results suggest that Fematrin-1 is involved in the fetomaternal cell-to-cell fusion in Bovinae placenta (Fig. 3) and the newly acquired endogenous retroelement has contributed to generating placentation diversity through ruminant evolution.
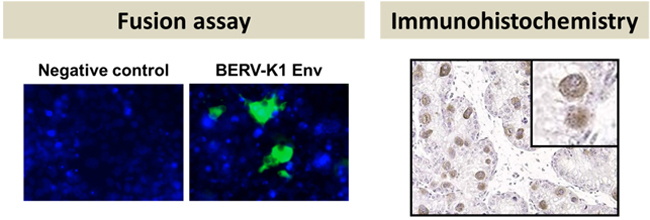 Fig. 1: Fusion assay (left panel) and immunohistochemistry (right panel) of BERV-K1 Env. Fused cells are visualized as green foci in the fusion assay. BERV-K1 Env was revealed to be specifically expressed in BNCs by immunohistochemistry. |
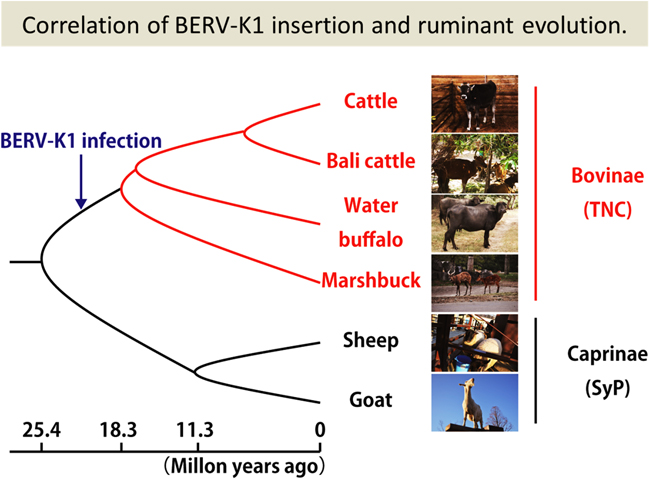 Fig. 2: Correlation of BERV-K1 insertion and ruminant evolution. The arrow indicates the estimated point of BERV-K1 integration into bovines. |
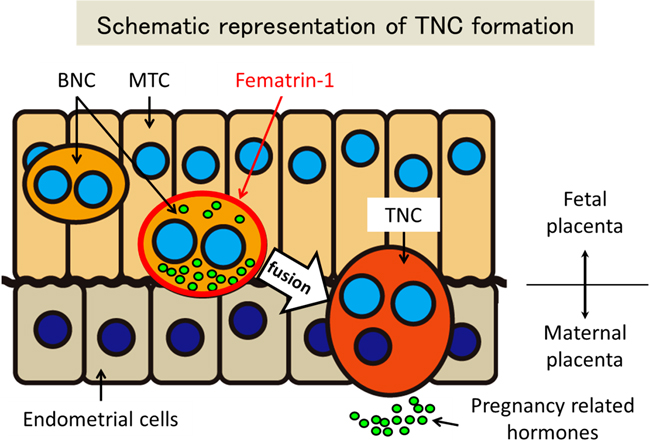 Fig. 3: Schematic representation of the mechanisms for TNC formation. BNCs are differentiated from MTC and express Fematrin-1. Then, BNCs fuse with maternal endometrial cells and form TNCs. TNCs are considered to facilitate to transfer pregnancy related hormones, which are produced by BNCs, to maternal blood stream. |
This study was supported by the Programme for Promotion of Basic and Applied Research for Innovations Bio-oriented Industry, Ministry of Agriculture, Forestry, and Fisheries of Japan. Yuki Nakaya is currently and So Nakagawa was supported by grants-in-aid from the Japanese Society for the Promotion of Science Fellows.
Paper information
[DOI] http://dx.doi.org/10.1128/JVI.01398-13
Yuki Nakaya, Katsuo Koshi, So Nakagawa, Kazuyoshi Hashizume, Takayuki Miyazawa.
Fematrin-1 is involved in fetomaternal cell-to-cell fusion in Bovinae placenta and contributed to diversity of ruminant placentation.
Journal of Virology, JVI.01398-13; published ahead of print 17 July 2013,

