Flippase is endocytosed following Ca2+ mediated PKC activation
In a new study published in Nature Communications , researchers from the school of Pharmaceutical Sciences led by Hye-Won Shin found a new regulatory mechanism that maintains the asymmetry of the two layers making up the cell membrane. Moreover, this mechanism is controlled by a well-studied molecular pathway, opening the door for possible future drug targets.
The cell membrane -- or plasma membrane -- is made up of two layers of compounds called phospholipids. Many phospholipids form the membrane, each with a slightly different chemical makeup that allows for the cell to detect and react to different stimuli.
One such phospholipid is Phosphatidylserine (PS), and is found mostly on the inside surface of the cell. Research has shown that PS exposed to the outer surface of the cell is critical for several biological processes including: cell death, platelet coagulation, immune response, and fusion of muscle cells. Therefore, controlling which side of the cell PS remains on is crucial. This is where "flippases" come in.
True to their name, flippases "flip" phospholipids from facing the outside of the cell to the inside. The Shin team previously found that a flippase called ATP11C controls PS localization. However, they still could not find how it is regulated in response to specific signals in a living cell.
The team conducted a series of flippase assays to measure the activity of ATP11C, while observing its actions with live imaging analysis. They found that when calcium levels in the cell increased, ATP11C was transported to the inside of the cell, essentially sequestering it from the membrane.
The team further demonstrated that this increase in calcium is triggered by a series of well-studied signaling proteins called G-protein coupled receptors -- or GPCRs. Specifically, when receptors for serotonin and histamine were activated, calcium levels in the cell increased and triggered the network that transports ATP11C. Once the signaling stopped, the flippase was recycled back to the membrane.
Shin explains that this is the first such evidence of signal-dependent regulation of a mammalian flippase. This new insight could lead to a better understanding of how living cells regulate PS exposure and recovery while interacting with the environment. Moreover, GPCRs are targeted by at least 30% of all drugs. These new findings could be used to develop new drugs for GPCRs, including for serotonin and histamine receptors.
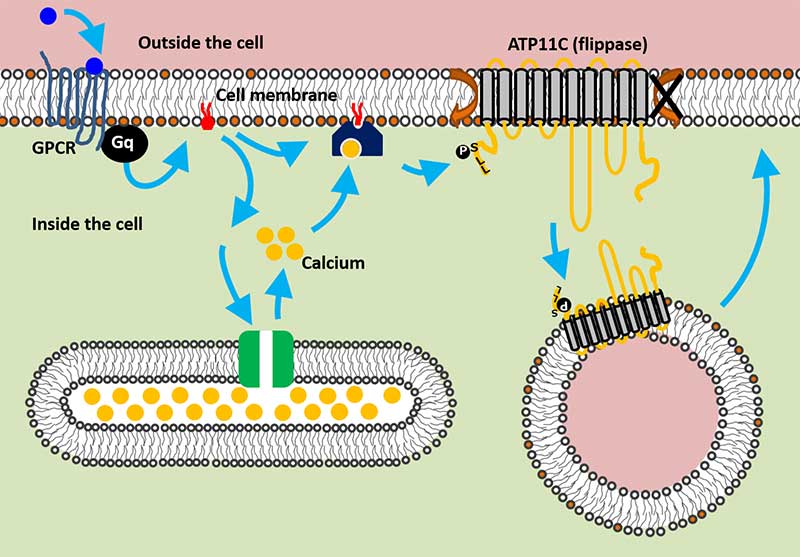
GPCR signaling pathway regulates ATP11C activity (Shin Lab, Kyoto University)
Paper Information
【DOI】 https://doi.org/10.1038/s41467-017-01338-1
【KURENAI ACCESS URL】 http://hdl.handle.net/2433/227907
Hiroyuki Takatsu, Masahiro Takayama, Tomoki Naito, Naoto Takada, Kazuya Tsumagari, Yasushi Ishihama, Kazuhisa Nakayama & Hye-Won Shin (2017). Phospholipid flippase ATP11C is endocytosed and downregulated following Ca2+-mediated protein kinase C activation. Nature Communications, 8, 1423.





