Water's peculiar properties derive from its ability to form hydrogen bonds with many neighboring water molecules. However, the difficulty in characterizing the complexity of these networks means it is beneficial to be able to study just two water molecules and how they bind together. Soccer-ball-shaped carbon cages, called fullerenes, offer an option for achieving this. A single water molecule had already been introduced into a fullerene made up of 60 carbon atoms, but recent work by Kyoto University researchers has resulted in two water molecules being trapped inside a 70-carbon-atom fullerene, the space inside which is only around four ten-billionths of a meter across.
As recently reported online in the journal Nature Chemistry , using a method they call "molecular surgery", the team formed a hole in a large carbon-based cage and trapped two water molecules inside it. This let them study how these molecules interacted, free of disturbance from other factors. The new approach could boost understanding of how molecules behave at the molecular level.
The molecular surgery approach used in this study involved breaking some of the bonds between the cage's carbon atoms, using high pressure to force water into the cage via the newly formed hole, and then closing it again. The researchers found that a range of techniques could distinguish between carbon cages containing zero, one, or two water molecules, as well as characterize how the two molecules behaved in this unusual environment. Specifically, it was found that they formed a single hydrogen bond between them, which repeatedly broke and reformed. This new approach could be used for studying not only water dimers, but also other pairs of molecules and their interactions in isolation.
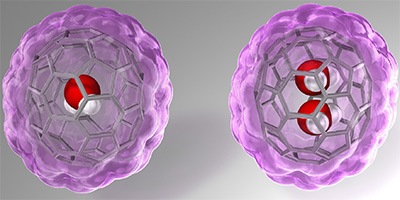
Kyoto University researchers have enabled the study of the bonding between two isolated water molecules by introducing the molecules into a hollow spherical carbon-based structure called fullerene C 70
Paper Information
[DOI] http://dx.doi.org/10.1038/nchem.2464
Rui Zhang, Michihisa Murata, Tomoko Aharen, Atsushi Wakamiya, Takafumi Shimoaka, Takeshi Hasegawa and Yasujiro Murata
"Synthesis of a distinct water dimer inside fullerene C70"
Nature Chemistry , 07 March 2016





