Cutting-Edge Research
in Kyoto University
STAT-PHYS
When Simple Liquids are not so Simple Simplest hydrogen liquid exhibits mysterious properties that never appear in ordinary liquids.
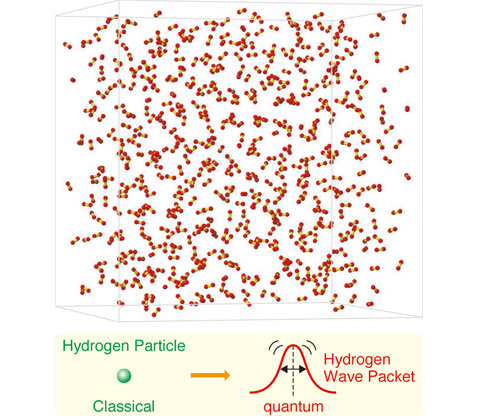
Hydrogen (H2) is the simplest of all molecular species. Defining a hydrogen nucleus, the lightest atom in the periodic table, is not straightforward. Instead of a “particle”, it is rather like a wave packet. Actually, this simplest atom exhibits strong nuclear quantum effects (NQEs) ? the nucleus cannot stop “beating” and can be spatially delocalized. Such NQEs of hydrogen nuclei in an H2 liquid dominate the structure and thermodynamic properties, making it mysterious. Liquids exhibiting NQEs are called quantum liquids, and show phenomena that have never been observed for ordinary classical liquids. Understanding microscopic molecular dynamics and the resulting anomalous properties of quantum liquids remains an open problem. I am elucidating unexplained anomalous properties of quantum liquids by developing a new computational method taking into account NQEs that can be applied to a many-molecule system with feasible computational cost. The developed method provides intuitive understandings of real-time dynamics of H2 molecules even in the liquid phase including its H-H bond vibrations, molecular orientations and librational motions.

- Kim Hyeon-Deuk, PhD
- Assistant Professor,
Graduate School of Science
