
From left, Prof Tsumaki and Researcher Okada
Skeletal dysplasia describe hundreds of disorders that affect bone and cartilage. One example is type II collagenopathy (COL2pathy), a disease associated with mutations in type II collagen, the primary molecule found in cartilage. Type II collagen is essential for the extracellular matrix, which provides strength in structures exposed to high mechanical load. At the molecular level, COL2pathy is stereotyped by mutations in the type II collagen gene COL2A1, which is associated with misfolding of the collagen protein and increased ER stress signaling in the cell.
To study the molecular mechanism of COL2pathy, the Proffesor Noriyuki Tsumaki team at the Center of iPS Research and Application (CiRA) used three reprogramming methods to generate chrondocytes using fibroblasts from COL2pathy patients: direct reprogramming of fibroblasts into chrondocytes (iChon cells), the generation of COL2pathy-iPS cells that were then differentiated into chrondocytes, and the generation of teratomas by transplanting those iPS cells into immunodeficient mice. They found independent of the method that the reprogrammed cells could express type II collagen, but suffered from excess ER stress, which promoted cell apoptosis and suggested misfolding of the type II collagen. Consistent with those observations, they also found that cartilage in teratoma formed by patient-specific iPS cells accumulated type II collagen within the cells rather than in the extracellular space (Fig).
Ironically, the addition of chondrogenic growth factors risks aggravating the apoptosis, since it would promote the expression of type II collagen including mutant type II collagen, which would in turn increase ER stress. The researchers confirmed this hypothesis by adding ascorbic acid to cell cultures, which increased the abnormalities in diseased cells and activation of the ER stress signaling. Alternatively, the team investigated the benefits of trimethylamine N-oxide (TMAO), a small chaperone that targets unfolded proteins. Researcher Minoru Okada, the first author of the report, explains, "two papers showed positive effects for TMAO, one on chrondrocyte maturation and one on ER stress". Okada found that TMAO increased the secretion of type II collagen while decreasing the stress signaling and the apoptosis to some extent. The exact effects of TMAO, however, for example, whether they actually correct the misfolding, still remain to be elucidated. "We are now looking for more compounds that substantially rescue phenotypes of COL2pathy-reprogrammed cells and studying the mechanism of the effect", says Okada.
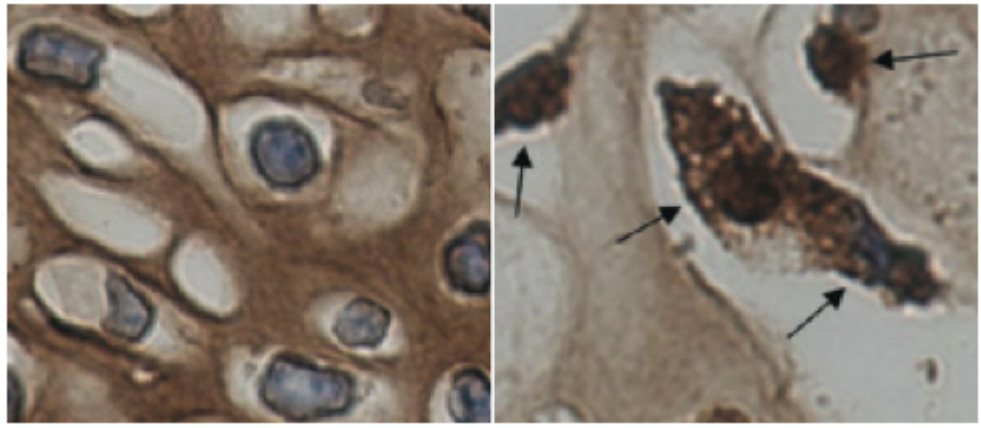
Figure. Type II collagen disperses differently in healthy and COL2pathy cells. iPS cells generated from healthy and COL2pathy patients were injected into immunodeficient mice to form tetatromas. Type II collagen was localized in the ECM of healthy cells (left, brown), but existed in the cytoplasm of diseased cells (right, arrows).
Paper information
[DOI] http://dx.doi.org/10.1093/hmg/ddu444
Minoru Okada, Shiro Ikegawa, Miho Morioka, Akihiro Yamashita, Atsushi Saito, Hideaki Sawai, Jun Murotsuki, Hirofumi Ohashi, Toshio Okamoto, Gen Nishimura, Kazunori Imaizumi, and Noriyuki Tsumaki
"Modeling type II collagenopathy skeletal dysplasia by directed conversion and induced pluripotent stem cells"
Human Molecular Genetics , first published online: September 3, 2014





