In recent years, attempts to utilize genetic analysis in clinical practice have intensified with the objective of making it possible for clinicians to apply patients' genetic information in respect of positive or negative effects of particular drugs in determining the best use of drugs for each patient, which could lead to prevention of adverse effects. The challenge lies in enabling clinicians to deal with huge amounts of genetic information and interpret it correctly in actual clinical settings.
Professor Fumihiko Matsuda of the Center for Genomic Medicine, Graduate School of Medicine, Kyoto University, and Toyo Kohan Co., Ltd. have jointly addressed the challenge and successfully developed a genetic analysis kit that allows treatment to be tailored to patients (personalized medicine) in thyroid medication. This research was undertaken as one of Kyoto University's Center for Innovation (COI) Programs.
The genetic analysis kit, which uses Toyo Kohan's biotip technology, enables determination and measurement of four genetic polymorphisms related to agranulocytosis, which is an adverse effect of the antithyroid drug Thiamazole, by means of a simple method with a high degree of accuracy in actual clinical practice. Results achieved with this kit indicate that risks of adverse effects can be effectively assessed, which will bring significant benefits to patients and help clinicians in practice.
This breakthrough development will be implemented in specialized hospitals as a kit for research purposes. Meanwhile, ongoing studies will be conducted with a view to developing genetic analysis kits to predict adverse effects caused by medications for other medical conditions, and other kits to provide useful information that support healthy living, in line with the aim of the COI program, which is "to build a smart, flexible, and accommodating society".
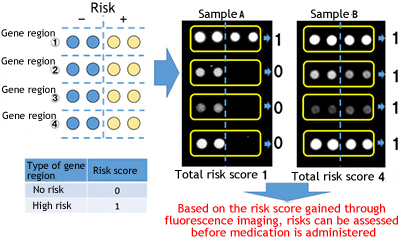
Risk assessment using the genetic analysis kit
This genetic analysis kit makes it possible to determine how many genetic polymorphisms related to adverse effects the patient has. (Risk scores range from 0 to 4; the bigger the number, the higher the risk.)





