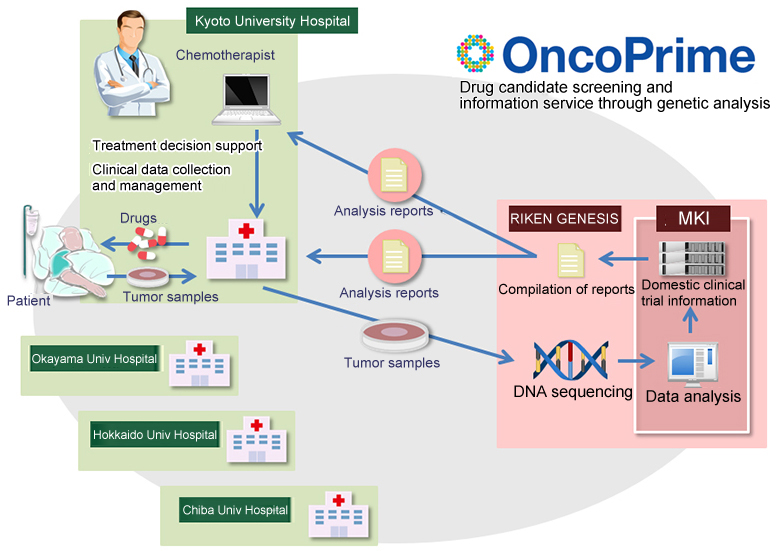
In April 2015, Kyoto University Hospital became the first Japanese institution to offer clinical sequencing tests to cancer patients. This service, called OncoPrime, comprehensively analyzes gene mutations associated with cancer in each patient to aid treatment and enable precision medicine.
KU Hospital has provided sequencing to over 150 patients to date -- those with cancers of unknown primary (CUP), rare cancers, and cancers unresponsive to standard therapies. The service is also available at three other university hospitals -- Okayama, Hokkaido, and Chiba -- and is being prepared or considered for implementation at several others.
The sequencing and analysis are handled by Mitsui Knowledge Industry Co, Ltd (MKI), at a facility specially built to comply with the stringent quality-control requirements of the Clinical Laboratory Improvement Amendments (CLIA) * .
MKI has now partnered with RIKEN GENESIS Co, Ltd to launch a new service, OncoPrime Basic, in which the latter performs the sequencing. The service will be available from all the four university hospitals, beginning with KU Hospital. This plan is expected to help make clinical sequencing less expensive and time-consuming over the coming years.
KU Hospital's next goal is to have clinical sequencing designated as an "advanced medical procedure" by the health ministry, a goal it hopes to achieve by consistently demonstrating the effectiveness of the technique and working closely with other medical institutions.
CLIA is a US regulatory standards program applicable to all clinical laboratory testing performed on humans in the country. Accredited facilities are subject to periodic inspections to ensure quality control.